00:00-00:07
[Trulicity logo; ambient music plays]
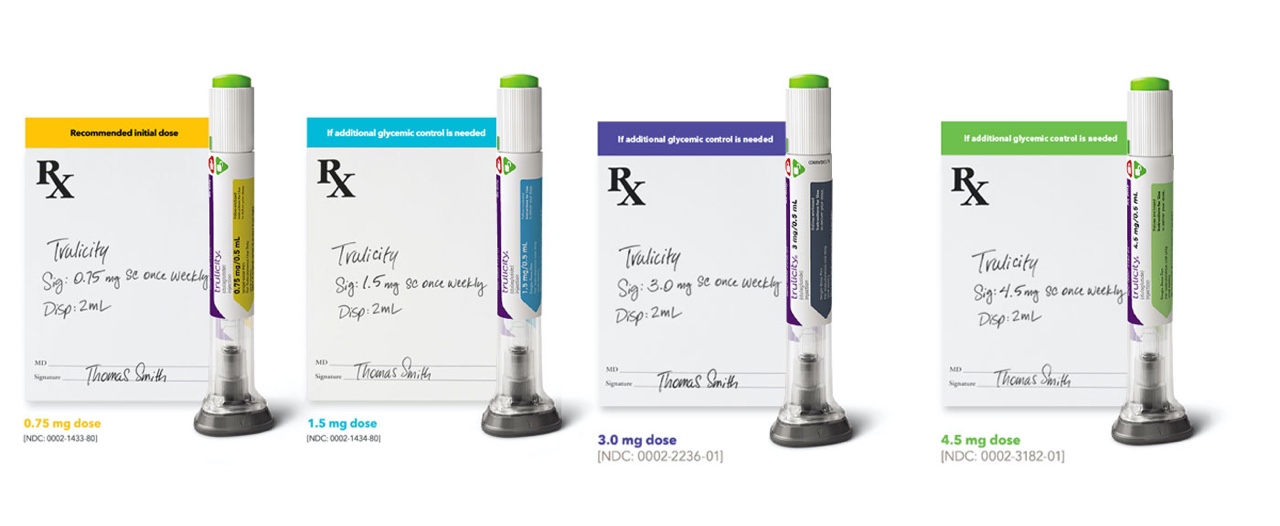
CAPTION: Once weekly Trulicity® (dulaglutide) injection 0.75 mg/0.5mL, 1.5mg/0.5mL, 3.0mg/0.5mL, 4.5mg/0.5mL
CAPTION: All subjects are paid representatives of Lilly, and are expressing their own opinions.
CAPTION: See accompanying Prescribing Information, including boxed warning about possible thyroid tumors, including thyroid cancer, provided along with this video.
00:07-00:21
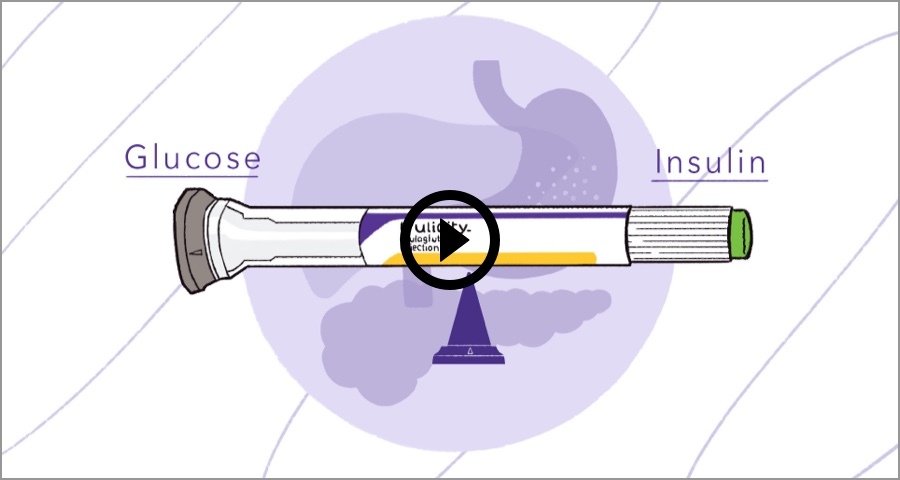
CAPTION: Indication: Trulicity is a glucagon-like peptide-1 receptor agonist (GLP-1 RA) that is indicated as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients 10 years of age and older with type 2 diabetes mellitus and to reduce the risk of major adverse cardiovascular events (cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) in adults with type 2 diabetes mellitus who have established cardiovascular disease or multiple cardiovascular risk factors.
CAPTION: Limitations of Use: Has not been studied in patients with a history of pancreatitis; consider other antidiabetic therapies in these patients. Not for the treatment of type 1 diabetes mellitus. Has not been studied in patients with severe gastrointestinal disease, including severe gastroparesis and is therefore not recommended in these patients.
CAPTION: See accompanying Prescribing Information, including boxed warning about possible thyroid tumors, including thyroid cancer, provided along with this video.
00:21-00:42
CAPTION: WARNING: RISK OF THYROID C-CELL TUMORS: In male and female rats, dulaglutide causes a dose-related and treatment-duration-dependent increase in the incidence of thyroid C-cell tumors (adenomas and carcinomas) after lifetime exposure. It is unknown whether Trulicity causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of dulaglutide-induced rodent thyroid C-cell tumors has not been determined.
CAPTION: Trulicity is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with Multiple Endocrine Neoplasia syndrome type 2, and in patients with a serious hypersensitivity reaction to dulaglutide or any of the product components.
CAPTION: See accompanying Prescribing Information, including boxed warning about possible thyroid tumors, including thyroid cancer, provided along with this video.
00:42-00:57
[Exterior shot of Four Seasons Nursing & Rehabilitation Center; Jonathan S. speaks off camera; Jonathan S. talks to camera, sitting on couch in an office]
JONATHAN S.: Long-term care is interesting. These are our grandmothers. These are parents that I look at. And we form certain bonds with them because some of them may be abandoned by their families, so we become their families. We take care of them.
CAPTION: Jonathan Shaatal, MS, RPh, FASCP Director of Pharmacy – Four Seasons Nursing & Rehabilitation Center
00:57-01:03
[Shots of hallway at long-term care facility; staff members help a resident walk down the hallway, interact with residents, work on a computer]
01:03-01:14
[Jonathan S. talks to camera; shot of sign at facility that reads, “Recover Restore Rejuvenate at Four Seasons Rehab”; shot of exercise and rehab equipment]
JONATHAN S.: In the last couple of years, the patients arriving to our facility usually have this triad of disease states: hyperlipidemia, hypertension, and diabetes.
01:14-01:37
[Dr. Daniel C. sits on couch in office, talking to camera; Dr. Daniel C. works on computer, looking at patient records]
DR. DANIEL C.: The prevalence of diabetes is 25%, similar to the outside population. But over the long-term care, I believe that it’s more. And the thing is that with insulin, the patients may refuse insulin because of the discomfort. So that's three, four injections a day. Plus, the coverage whenever the sugars are way up.
CAPTION: Dr. Daniel Cimafranca, MD Physician – Four Seasons Nursing & Rehabilitation Center
CAPTION: The prevalence of diabetes in adults over 65 is approximately 25% Source: https://www.cdc.gov/diabetes/data/statistics-report/diagnosed-undiagnoseddiabetes.html. Accessed September 15, 2022.
01:37-01:46
[Shot of Dr. Daniel C. and two other staff members working at nurses’ station]
DR. DANIEL C.: And I would say about maybe two years ago, we started to get some pushback from our nursing staff as well as the patients.
01:46-02:11
[Voiceover by Floremar D.; hallway shot; Floremar D. talks to camera, talks to nursing staff member]
FLOREMAR D.: At Four Seasons, we're a 270-bed nursing home facility located in Brooklyn. Take into consideration having to cover 45 patients with their testing blood sugar levels for patients, whether they have to be monitored twice, daily, three times daily, before and after meals. It is challenging, and it is a burden for the nurse to be able to work on her time schedule.
CAPTION: Floremar Dulinayan, MS, RN, WCC, RAC-CT Director of Nursing – Four Seasons Nursing & Rehabilitation Center
02:11-02:21
[Jonathan S. walks down the hallway, holding a large binder]
JONATHAN S.: We needed to think, Is there another treatment modality that we can bring to Four Seasons nursing home to treat their diabetes?
02:21-02:38
[Jonathan S. and Floremar D. speak to nurses; nurse holds a Trulicity pen, demonstrates how to use it; Floremar D. talks to camera]
FLOREMAR D.: Transitioning our patients from daily injections to once weekly, it has kind of like lifted the burden of the nurses. The other advantage with using Trulicity is its ease of use.
02:38-02:58
[Jonathan S. talks to camera; Jonathan S. shows Trulicity pen to a staff member working at a computer]
JONATHAN S.: The simplicity, the ease of use. Keep in consideration that many of our patients sometimes will skip meals. Sometimes our patients will not eat their whole total meal. And as a result, we're also worried about hypoglycemia. What we like about the Trulicity is that it works in a glucose dependent environment.
02:58-03:08
[Close-up of Trulicity pen; Dr. Daniel C. talks to camera; shot of patient’s legs and feet]
DR. DANIEL C.: The lesser the medications, the lesser the pain for the patient, the better would be the adherence and subsequently the better the result would be.
03:08-03:15
[Jonathan S. talks to camera]
JONATHAN S.: We now see that many of our patients are achieving their individualized hemoglobin A1C goal.
03:15-03:35
[Floremar speaks to nurses; shot of facility gathering room, painted and decorated with trees and flowers; exterior shot of Four Seasons]
FLOREMAR D.: For something as simple as reducing the amount and frequency of medication administration, it allows the nurses more time to listen, more time to make our patients feel how much we care. And knowing you have made a difference with those whose wisdom has come with time.
03:35-03:42
[Trulicity logo; ambient music plays]
CAPTION: Once weekly Trulicity® (dulaglutide) injection 0.75 mg/0.5mL, 1.5mg/0.5mL, 3.0mg/0.5mL, 4.5mg/0.5mL
CAPTION: All subjects are paid representatives of Lilly, and are expressing their own opinions.
03:42-03:57
CAPTION: Limited data with Trulicity in pregnant women are not sufficient to determine a drug associated risk for major birth defects and miscarriage. Based on animal reproduction studies, there may be risks to the fetus from exposure to dulaglutide. Use only if potential benefit justifies potential risk to fetus.
CAPTION: There are no data on the presence of dulaglutide in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Trulicity and any potential adverse effects on the breastfed infant from Trulicity or from the underlying maternal condition.
CAPTION: Safety and effectiveness of Trulicity have not been established and use is not recommended in patients less than 18 years of age .
CAPTION: For more information visit 1-844-TRU-INFO or Trulicity.com
03:57-04:35
NARRATOR: Trulicity is a glucagon like peptide 1 receptor agonist (GLP-1 RA) that is indicated as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients 10 years of age and older with type 2 diabetes mellitus and to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) in adults with type 2 diabetes mellitus who have established cardiovascular disease or multiple cardiovascular risk factors.
CAPTION: MAJOR STATEMENT OF RISK FOR TRULICITY: Trulicity is a glucagon like peptide 1 receptor agonist (GLP-1 RA) that is indicated as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients 10 years of age and older with type 2 diabetes mellitus and to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) in adults with type 2 diabetes mellitus who have established cardiovascular disease or multiple cardiovascular risk factors.
04:35-04:56
NARRATOR: Trulicity has not been studied in patients with a history of pancreatitis; consider another antidiabetic therapy. Not for the treatment of type 1 diabetes mellitus. Has not been studied in patients with severe gastrointestinal disease, including severe gastroparesis and is therefore not recommended in these patients.
CAPTION: Trulicity has not been studied in patients with a history of pancreatitis; consider another antidiabetic therapy. Not for the treatment of type 1 diabetes mellitus. Has not been studied in patients with severe gastrointestinal disease, including severe gastroparesis and is therefore not recommended in these patients.
04:56-05:26
NARRATOR: WARNING: RISK OF THYROID C-CELL TUMORS: In male and female rats, dulaglutide causes a dose-related and treatment-duration-dependent increase in the incidence of thyroid C-cell tumors (adenomas and carcinomas) after lifetime exposure. It is unknown whether Trulicity causes thyroid C-cell tumors, including medullary thyroid carcinoma, or MTC, in humans as human relevance of dulaglutide-induced rodent thyroid C-cell tumors has not been determined.
CAPTION: WARNING: RISK OF THYROID C-CELL TUMORS: In male and female rats, dulaglutide causes a dose-related and treatment-duration-dependent increase in the incidence of thyroid C-cell tumors (adenomas and carcinomas) after lifetime exposure. It is unknown whether Trulicity causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of dulaglutide-induced rodent thyroid C-cell tumors has not been determined.
05:26-05:43
NARRATOR: Trulicity is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with Multiple Endocrine Neoplasia syndrome type 2, and in patients with a serious hypersensitivity reaction to dulaglutide or any of the product components.
CAPTION: Trulicity is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with Multiple Endocrine Neoplasia syndrome type 2, and in patients with a serious hypersensitivity reaction to dulaglutide or any of the product components.
05:43-06:01
NARRATOR: Pancreatitis: Has been reported in clinical trials. Discontinue promptly if pancreatitis is suspected and initiate appropriate management. Do not restart if pancreatitis is confirmed. Consider other antidiabetic therapies in patients with history of pancreatitis.
CAPTION: Pancreatitis: Has been reported in clinical trials. Discontinue promptly if pancreatitis is suspected and initiate appropriate management. Do not restart if pancreatitis is confirmed. Consider other antidiabetic therapies in patients with history of pancreatitis.
06:01-6:33
NARRATOR: Hypoglycemia: Patients receiving Trulicity in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. The risk of hypoglycemia may be lowered by a reduction in the dose of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
CAPTION: Hypoglycemia: Patients receiving Trulicity in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. The risk of hypoglycemia may be lowered by a reduction in the dose of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
06:33-06:54
NARRATOR: Hypersensitivity Reactions: Serious hypersensitivity reactions (for example, anaphylactic reactions and angioedema) have occurred. Discontinue Trulicity and promptly seek medical advice. Trulicity is contraindicated in patients with a previous serious hypersensitivity reaction to dulaglutide or to any of the components of Trulicity.
CAPTION: Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., anaphylactic reactions and angioedema) have occurred. Discontinue Trulicity and promptly seek medical advice. Trulicity is contraindicated in patients with a previous serious hypersensitivity reaction to dulaglutide or to any of the components of Trulicity.
06:54-07:03
NARRATOR: Acute Kidney Injury: Monitor renal function in patients with renal impairment reporting severe adverse gastrointestinal reactions.
CAPTION: Acute Kidney Injury: Monitor renal function in patients with renal impairment reporting severe adverse gastrointestinal reactions.
07:03-07:17
NARRATOR: Severe Gastrointestinal Disease: Use may be associated with gastrointestinal adverse reactions, sometimes severe. Has not been studied in patients with severe gastrointestinal disease and is not recommended in these patients.
CAPTION: Severe Gastrointestinal Disease: Use may be associated with gastrointestinal adverse reactions, sometimes severe. Has not been studied in patients with severe gastrointestinal disease and is not recommended in these patients.
07:17-07:28
NARRATOR: Diabetic Retinopathy Complications: Have been reported in a cardiovascular outcomes trial. Monitor patients with a history of diabetic retinopathy.
CAPTION: Diabetic Retinopathy Complications: Have been reported in a cardiovascular outcomes trial. Monitor patients with a history of diabetic retinopathy.
07:28-07:36
NARRATOR: Acute Gallbladder Disease: If cholelithiasis or cholecystitis are suspected, gallbladder studies are indicated.
CAPTION: Acute Gallbladder Disease: If cholelithiasis or cholecystitis are suspected, gallbladder studies are indicated.
07:36-07:53
NARRATOR: The most common adverse reactions: Incidence reported in ≥5% of patients treated with Trulicity were: nausea, diarrhea, vomiting, abdominal pain, decreased appetite, dyspepsia and fatigue.
CAPTION: The most common adverse reactions: Incidence reported in ≥5% of patients treated with Trulicity were: nausea, diarrhea, vomiting, abdominal pain, decreased appetite, dyspepsia and fatigue.
07:53-08:07
NARRATOR: Oral Medications and Delayed Gastric Emptying: Trulicity slows gastric emptying and may impact absorption of concomitantly administered oral medications.
CAPTION: Oral Medications and Delayed Gastric Emptying: Trulicity slows gastric emptying and may impact absorption of concomitantly administered oral medications.
CAPTION: DG HCP MSR 17NOV2022
CAPTION: Trulicity® and its delivery device base are registered trademarks owned or licensed by Eli Lilly and Company, its subsidiaries, or affiliates. Trulicity is available by prescription only.
CAPTION: PP-DG-US-4833 02/2023 ©Lilly USA, LLC 2022. All rights reserved.